Establishment of an infectious disease in a population requires more than just introduction of a pathogen into the population. It involves a complex interaction between the disease agent, the host, sometimes vector, and their environment. Understanding the ecological processes that determine the distribution, dynamics, severity, and evolution of diseases is the domain of the new science of Disease Ecology of Infectious Diseases.
The central theme of my research program is that the application of an ecological approach is crucial for understanding the processes by which anthropogenic environmental change leads to the emergence of zoonotic diseases. It is also crucial for identifying and targeting weak links in the transmission chain of such pathogens. My research approach combines empirical research involving field and laboratory work as well as theoretical work involving mathematical modeling, individual-based simulations, and conceptual models.
Research
Current research focuses on the following themes:
- The effect of anthropogenic land use change on disease emergence, with model systems include (a.) Cutaneous Leishmaniasis in the Middle East and (b.) La Crosse Encephalitis in western North Carolina.
- Identifying and targeting weak links in the transmission cycle of Leishmania major (Cutaneous Leishmaniasis agent):
- Oviposition ecology of sand flies: basic science and implications for attractant-based control.
- Systemic control using the rodent host as a trojan horse delivering the insecticide to the vector-host contact point
- Study of the spread of Lyme disease from Virginia to North Carolina: patterns and mechanisms
- Oviposition ecology of mosquitoes: effects of conspecifics, bacteria, and natural enemies
- Chiggers as potential vectors of Rickettsial pathogens in North Carolina
- Vector-host coupling: studying the epidemiological consequences of vector’s dependency on the host as a breeding resource using Individual-based modeling
La Crosse encephalitis (LACE) is a neurodegenerative viral pediatric disease transmitted by Aedes mosquitoes in the Appalachian mountain range. It is characterized by a highly focal distribution with vector population being highly fragmented given the topographic nature of the area. The incidence of LACE has increased in the greater Appalachian region G. Wasserberg in Collaboration with B. Byrd from Western Carolina University are researching the putative causal association between anthropogenic landscape change and LACE emergence. In a previous study, this work showed that poor peridomestic conditions enhance LACE risk by modifying the habitat use patterns of its mosquito vectors (one domestic: Aedes triseriatus and two invasives: A. japonicus and A. albopictus; Tamini et al. 2021). Current studies are evaluating the mosquito and virus distribution along forest-to-field ecotones (the predominant landscape feature in this region) and tested experimentally the effect of tire introduction on the distribution and population dynamics of the disease vectors at the horizontal and vertical dimensions (Schwarz 2021). Importantly, work has shown that vertical habitat features (i.e., tree canopy height) are important in this vector system (Schwarz et al. 2020).
- Experimental Landscape Epidemiology of La Crosse Virus in the Southern Appalachian Mountains. Ph.D., University of North Carolina at Greensboro Greensboro. Mentor: Gideon Wasserberg
- Schwarz, M., B. D. Byrd, B. F. Marayati, P. W. Blum, M. B. Wells, A. D. Greene, M. Taylor, and Wasserberg. 2020. Horizontal distribution affects the vertical distribution of native and invasive container-inhabiting Aedes mosquitoes within an urban landscape. J Vector Ecol 45: 16-24.
- Tamini, T., B. Byrd, A. Goggins, C. Sither, L. White, and Wasserberg. 2021. Peridomestic conditions affect La Crosse virus entomological risk by modifying the habitat Peridomestic conditions affect La Crosse virus entomological risk by modifying the habitat Journal of Vector Ecology 46: 34-37.
- Wilson, R., B. Harrison, M. Riles, Wasserberg, and B. Byrd. 2014. Differential identification of Aedes triseriatus (Say) and Aedes hendersoni Cockerell (Diptera: Culicidae) by a novel duplex PCR assay. Journal of the American Mosquito Control Association 30: 79-82.
Leishmaniases are the third most pervasive insect-bone diseases (following Malaria and Dengue), affecting tropical as well as arid regions in under-developed countries. However, CL is a neglected disease and developing a more thorough understanding of its ecology is vital. We are using Old World Zoonotic Cutaneous Leishmaniasis (ZCL) as a model system to study this question. Leishmania major, the protozoan parasite that causes Old World ZCL, is transmitted by the sand fly Phlebotomus papatasi and maintained by Psammomys obesus (the fat sand rat) in native arid landscapes but recently was described to also be maintained by Meriones tristrami in agriculturally modified regions. In native arid regions, we have shown that anthropogenic disturbances, of various types, share the common effect of elevating soil moisture, which enhances sand fly and sand rat abundance and thereby amplifying pathogen transmission and spill-over rates of the infection into adjacent human populations (see, flow chart model below) (Wasserberg et al. 2002, 2003ab, Berger et al. 2014). However, a recent outbreak of Old World ZCL in a northern, mostly agricultural semi-arid region, is yet poorly understood. We are currently studying this system in order to better understand the ecological and, possibly, evolutionary processes underlying this emergence.
Wasserberg, G., Z. Abramsky, G. Anders, M. El-Fari, G. Schoenian, L. Schnur, B. P. Kotler, I. Kabalo, and A. Warburg. 2002. The ecology of cutaneous leishmaniasis in Nizzana, Israel: infection patterns
Berger, R., Wasserberg, A. Warburg, L. Orshan, and B. P. Kotler. 2014. Zoonotic disease in a peripheral population: persistence and transmission of Leishmania major in a putative sink-source system in the Negev Highlands, Israel. Vector Borne Zoonotic Diseases 14: 592-600.
Wasserberg, G., I. Yarom, and A. Warburg. 2003a. Seasonal abundance patterns of the sandfly Phlebotomus papatasi in climatically distinct foci of cutaneous leishmaniasis in Israeli deserts. Medical and Veterinary Entomology 17: 452-456.
Wasserberg, G., Z. Abramsky, B. P. Kotler, R. S. Ostfeld, I. Yarom, and A. Warburg. 2003b. Anthropogenic disturbances enhance occurrence of cutaneous leishmaniasis in Israel deserts: patterns and mechanisms. Ecological Applications 13: 868-881.
Humanity is facing an epidemiological transition, characterized by the resurgence and emergence of old and novel infectious diseases the majority of which are associated with a wildlife source (zoonoses) and/or arthropod vectors. Human-induced environmental changes such as encroachment into pristine environments, land-use change, and climate change has been implicated as key drivers of this phenomenon. For a transmission hot spot to become established, the ecological niches of the pathogen, host, and (for vector borne pathogens) the vector must overlap within a permissive environment. The specific subset of environmental conditions that enable the persistence of the transmission cycle is often referred to as the “disease niche” (figure below). Human encroachment into pristine environments may exposes humans to novel pathogens but it may also enhance transmission by improving the ecological conditions for some (or all) the ecologic components of the enzootic system. We showed that such small-scale anthropogenic effects have the potential to enhance transmission dynamics for both Old-World Cutaneous Leishmaniasis in Israel and for La Crosse Encephalitis in western North Carolina. However, large scale anthropogenic landscape modification may also have substantial effects on the structure and function of these systems. However, these are currently poorly understood.
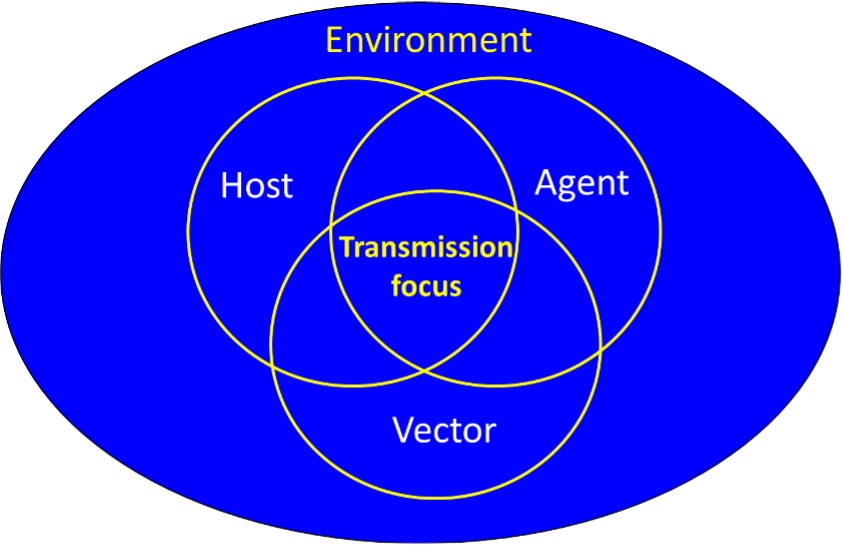
The disease niche conceptual model
1. The effect of anthropogenic land-use changes on the emergence and resurgence of Cutaneous Leishmaniasis in Israel
Leishmaniases are the third most pervasive insect-bone diseases (following Malaria and Dengue), affecting tropical as well as arid regions in under-developed countries. However, CL is a neglected disease and developing a more thorough understanding of its ecology is vital. We are using Old World Zoonotic Cutaneous Leishmaniasis (ZCL) as a model system to study this question. Leishmania major, the protozoan parasite that causes Old World ZCL, is transmitted by the sand fly Phlebotomus papatasi and maintained by Psammomys obesus (the fat sand rat) in native arid landscapes but recently was described to also be maintained by Meriones tristrami in agriculturally modified regions. In native arid regions, we have shown that anthropogenic disturbances, of various types, share the common effect of elevating soil moisture, which enhances sand fly and sand rat abundance and thereby amplifying pathogen transmission and spill-over rates of the infection into adjacent human populations (see, flow chart model below) (Wasserberg et al. 2002, 2003ab, Berger et al. 2014). However, a recent outbreak of Old World ZCL in a northern, mostly agricultural semi-arid region, is yet poorly understood. We are currently studying this system in order to better understand the ecological and, possibly, evolutionary processes underlying this emergence.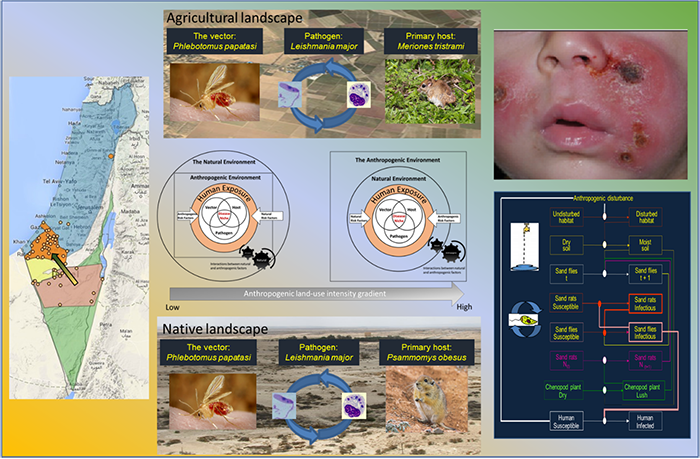
Berger, R., Wasserberg, A. Warburg, L. Orshan, and B. P. Kotler. 2014. Zoonotic disease in a peripheral population: persistence and transmission of Leishmania major in a putative sink-source system in the Negev Highlands, Israel. Vector Borne Zoonotic Diseases 14: 592-600.
Wasserberg, G., I. Yarom, and A. Warburg. 2003a. Seasonal abundance patterns of the sandfly Phlebotomus papatasi in climatically distinct foci of cutaneous leishmaniasis in Israeli deserts. Medical and Veterinary Entomology 17: 452-456.
Wasserberg, G., Z. Abramsky, B. P. Kotler, R. S. Ostfeld, I. Yarom, and A. Warburg. 2003b. Anthropogenic disturbances enhance occurrence of cutaneous leishmaniasis in Israel deserts: patterns and mechanisms. Ecological Applications 13: 868-881.
Wasserberg, G., Z. Abramsky, G. Anders, M. El-Fari, G. Schoenian, L. Schnur, B. P. Kotler, I. Kabalo, and A. Warburg. 2002. The ecology of cutaneous leishmaniasis in Nizzana, Israel: infection patterns 
2. The effect of anthropogenic land-use changes on the emergence and resurgence of La Crosse Encephalitis in western North Carolina.
La Crosse encephalitis (LACE) is a neurodegenerative viral pediatric disease transmitted by Aedes mosquitoes in the Appalachian mountain range. It is characterized by a highly focal distribution with vector population being highly fragmented given the topographic nature of the area. The incidence of LACE has increased in the greater Appalachian region G. Wasserberg in Collaboration with B. Byrd from Western Carolina University are researching the putative causal association between anthropogenic landscape change and LACE emergence. In a previous study, this work showed that poor peridomestic conditions enhance LACE risk by modifying the habitat use patterns of its mosquito vectors (one domestic: Aedes triseriatus and two invasives: A. japonicus and A. albopictus; Tamini et al. 2021). Current studies are evaluating the mosquito and virus distribution along forest-to-field ecotones (the predominant landscape feature in this region) and tested experimentally the effect of tire introduction on the distribution and population dynamics of the disease vectors at the horizontal and vertical dimensions (Schwarz 2021). Importantly, work has shown that vertical habitat features (i.e., tree canopy height) are important in this vector system (Schwarz et al. 2020).
Experimental Landscape Epidemiology of La Crosse Virus in the Southern Appalachian Mountains. Ph.D., University of North Carolina at Greensboro Greensboro. Mentor: Gideon Wasserberg
Schwarz, M., B. D. Byrd, B. F. Marayati, P. W. Blum, M. B. Wells, A. D. Greene, M. Taylor, and Wasserberg. 2020. Horizontal distribution affects the vertical distribution of native and invasive container-inhabiting Aedes mosquitoes within an urban landscape. J Vector Ecol 45: 16-24.
Tamini, T., B. Byrd, A. Goggins, C. Sither, L. White, and Wasserberg. 2021. Peridomestic conditions affect La Crosse virus entomological risk by modifying the habitat Peridomestic conditions affect La Crosse virus entomological risk by modifying the habitat Journal of Vector Ecology 46: 34-37.
Wilson, R., B. Harrison, M. Riles, G. Wasserberg, and B. Byrd. 2014. Differential identification of Aedes triseriatus (Say) and Aedes hendersoni Cockerell (Diptera: Culicidae) by a novel duplex PCR assay. Journal of the American Mosquito Control Association 30: 79-82.
Phlebotomine sand flies (Diptera: Psychodidae) transmit protozoan parasites (Leishmania spp.), as well as bacteria (Bartonella bacilliformis) and Phleboviruses. Most significant are the human leishmaniases. With no vaccine to protect against the etiologic agent, restriction of exposure to sand fly bites is the most effective preventive measure. Transmission prevention mainly involves personal protection (e.g., repellents, insecticide-treated clothing or bed-nets) and residual spraying with insecticides. However, the efficacy of the latter is decreasing due to the evolution of insecticide resistance. Furthermore, residual insecticide sprays also affect a wide range of non-target arthropods. Hence, a more focused, targeted, and efficient control method is urgently needed. With the rise of insect vector resistance to chemical pesticides and their effect on non-target species, there is an urgent need for the development of environment-friendly biologically based and targeted vector-control approaches. Utilizing a biological component of the vector’s biology to work against itself is considered as a hallmark of an efficacious approach because it is usually species-specific and less susceptible for the development of resistance. In my research, I target potential weak links that could potentially break the transmission chain of the Leishmania pathogens.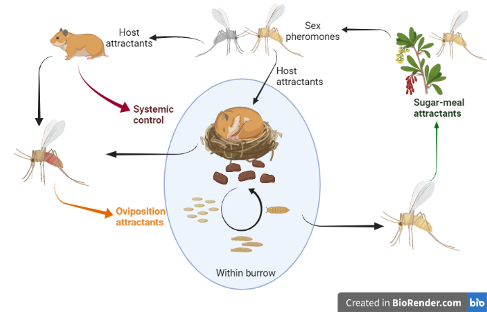
Life cycle of a sand fly species that is dependent on its blood-meal host for providing it with a breeding site and a shelter (termed – ‘tightly-coupled vector-host system’). Attractant-based intervention could target crucial aspects of its life cycle including sugar-meal attractants, sex pheromones, host cues, and oviposition attractants. My research (in this context) focuses, mainly, on oviposition attractants but also sugar-meal attractants. Feed-through systemic control uses the host to deliver the insecticide to the insect-host point of contact. This is also an important avenue my lab explores.
1. Oviposition attractants of sand flies: basic science and implications for control
An alternative approach to the delivery of the insecticide to the vector is to bring the vector to the insecticide using attractants. This attract-and-kill approach is commonly used to control disease vectors using volatile compounds such as sex pheromones, host odors, sugar meal sources, and bacterial mediated oviposition site attractants. In the context of controlling and surveilling disease vectors, oviposition-site attractants are expected to be particularly effective because they lure older females that have blood-fed at least once and are thus more likely to be infected with pathogens. Therefore, by targeting gravid females, control efforts can simultaneously reduce pathogen transmission and control population growth. Furthermore, since the response to oviposition cues is often species- or genus-specific, the impact on non-target species is expected to be minimal. For the same reason, the attractive lure can be integrated with a trap (e.g., CDC traps) for effective vector surveillance, providing a more precise estimate of the entomological risk of certain insect-borne pathogens in a given area. in addition, identifying oviposition stimulants can be important by facilitating sand fly mass rearing and transmission studies.
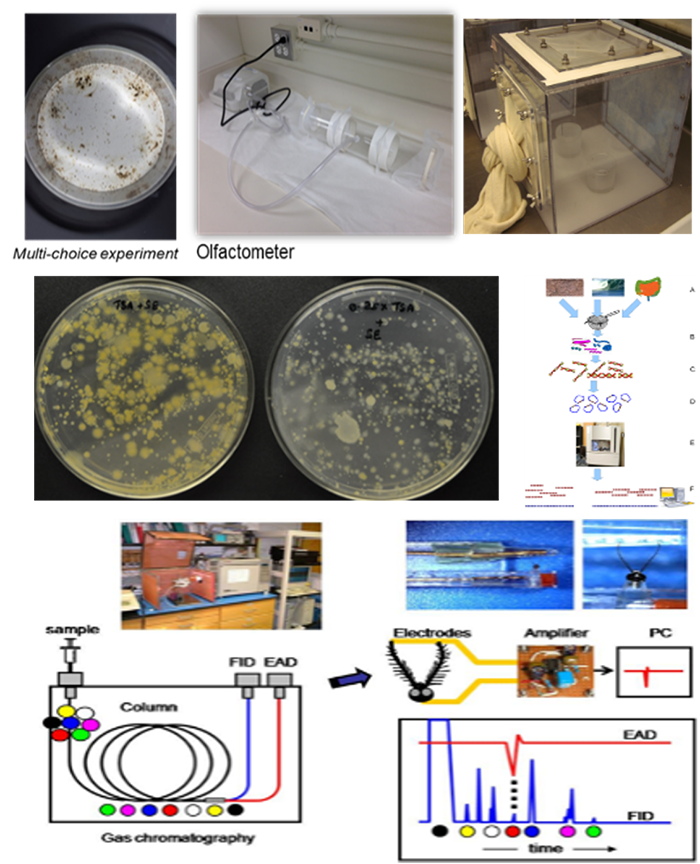
Unlike most biting Diptera, sand flies have a fully terrestrial life cycle. Eggs are typically laid in soil rich in organic material on which the coprophagic larvae feed and develop through four instars before pupation and adult emergence. During the past five years of an NIH-R01 funded, using an interdisciplinary project combining behavioral, electrophysiological, and microbiological studies (See, figure on the right), we made substantial contributions in advancing our understanding of sand fly oviposition biology. Among others, we optimized screening protocols of oviposition attractants and stimulants and characterized oviposition periodicity. Based on these bioassays, we identified a subset of highly attractive bacterial isolates, identified a range of highly attractive and oviposition stimulating compounds from bacterial and conspecific sources, characterized visual oviposition cues, described spatial skip oviposition of individual flies, identified egg hatching cues, and quantified the cost of autogenous oviposition.
Faw, L. R., K. Raymann, N. Romo Bechara, and Wasserberg. 2021. Larval Conditioning and Aging of Sand Fly Rearing Medium Affect Oviposition Site Selection in Phlebotomus papatasi (Diptera: Psychodidae) Sand Flies. J Med Entomol 58: 1931-1935.
Kakumanu, M. L., B. F. Marayati, C. Schal, C. Apperson, Wasserberg, and L. Ponnusamy. 2021a. Oviposition-Site Selection of Phlebotomus papatasi (Diptera: Psychodidae) Sand Flies: Attraction to Bacterial Isolates From an Attractive Rearing Medium. Journal of Medical Entomology 58: 518-527.
Kakumanu, M. L., B. F. Marayati, A. Katsumata, Wasserberg, C. schal, C. Apperson, and L. Ponnusamy. 2021b. Sphingobacterium phlebotosubstratum sp. nov., a new member of family Sphingobacteriaceae isolated from sand fly rearing media. International Journal of Systematic and Evolutionary Microbiology.
Mclaughlin, L., and Wasserberg. 2021. Spatial bet-hedging in sand fly oviposition: Factors affecting Skip oviposition in Phlebotomus papatasi sand flies. Vector borne and zoonotic diseases In press.
Nguyen, H. M., D. J. Kowacich, and Wasserberg. 2021. Temporal Bet-Hedging in Sand Fly Oviposition: Pharate Phlebotomus papatasi Sand Fly Neonates Regulate Hatching Time in Response to Organic Matter and Proximity to Conspecific Eggs. Vector Borne Zoonotic Dis 21: 275-279.
Kowacich, D., E. Hatano, C. Schal, L. Ponnusamy, C. S. Apperson, T. Shymanovich, and G. Wasserberg. 2020. The egg and larval pheromone dodecanoic acid mediates density-dependent oviposition of Phlebotomus papatasi. Parasit Vectors 13: 280.
Shymanovich, T., N. Hajhashemi, and Wasserberg. 2020. Quantitative and Qualitative Costs of Autogeny in Phlebotomus papatasi (Diptera: Psychodidae) Sand Flies. J Med Entomol 57: 852-861.
Shymanovich, T., L. Faw, N. Hajhashemi, J. Teague, C. Schal, L. Ponnusamy, C. S. Apperson, E. Hatano, and Wasserberg. 2019. Diel periodicity and visual cues guide oviposition behavior in Phlebotomus papatasi, vector of old-world cutaneous leishmaniasis. PLoS Negl Trop Dis 13: e0007165.
Marayati, B. F., C. Schal, L. Ponnusamy, C. S. Apperson, T. E. Rowland, and Wasserberg. 2015. Attraction and oviposition preferences of Phlebotomus papatasi (Diptera: Psychodidae), vector of Old-World cutaneous leishmaniasis, to larval rearing media. Parasit.Vectors 8: 663.
Wasserberg, G., and E. D. Rowton. 2011. Sub-additive effect of conspecific eggs and frass on oviposition rate of Lutzomyia longipalpis and Phlebotomus papatasi. Journal of Vector Ecology 36: S138-S143.
2. Attraction of old-World and New World sand flies to sugar-meal of varous sources
A 3-chamber in-line olfactometer designed for use with sand flies was used as a high-throughput method to screen honeys for attractiveness to Phlebotomus papatasi (four geographic isolates), P. duboscqi (two geographic isolates), and Lutzomyia longipalpis maintained in colonies at the Walter Reed Army Institute of Research. A diversity of unifloral honey odors were evaluated as a proxy for the natural floral odors that sand flies may use in orientation to floral sugar sources in the field. In the 3-chamber in-line olfactometer, the choice modules come directly off both sides of the release area instead of angling away as in the Y-tube olfactometer. Of the 25 honeys tested, five had a significant attraction for one or more of the sand fly isolates tested. This olfactometer and high-throughput method has utility for evaluating a diversity of natural materials with unknown complex odor blends that can then be down-selected for further evaluation in wind tunnels and/or field scenarios.
Wasserberg, G., P. Kirsch, and E. D. Rowton. 2014. Orientation of colonized sand flies Phlebotomus papatasi, P. duboscqi, and Lutzomyia longipalpis (Diptera: Psychodidae) to diverse honeys using a 3-chamber in-line olfactometer. Journal of Vector Ecology 39: 94-102.

3. Feed-through systemic control using the rodent host as a trojan horse for delivering the insecticide to the vector-host contact point.The strong dependency of some vectors on their host as a source of habitat can be viewed as a weak link in pathogen’s transmission cycles using the vertebrate host as a ‘Trojan horse’ to deliver insecticides directly to the vector-host point of contact (hereafter ‘feed-through systemic control’). This could, simultaneously, affect the survival of blood-feeding females and coprophagic larvae. Sand-flies, vectors of leishmaniasis worldwide, are often dependent on their bloodmeal host as a source of habitat and may therefore be good candidates for systemic control. In a preliminary collaborative study, we showed that Imidacloprid treated food pellets are effective in reducing larval survival that fed on treated rodent’s feces and on female feeding on treated blood. In a collaborative study with Dr. Ido Tsurin from the Ben-Gurion University, Israel, we deployed fipronil-treated baits in Meriones crassus burrows and demonstrated 86% reduction in the abundance of female sand-flies that exit burrows of Fipronil-treated jirds. We then, tested this approach at a larger town-level scale in the Negev desert of Israel, and demonstrated that this intervention significantly reduced L. major transmission rate.
Wasserberg, G., R. Poche, D. Miller, M. Chenault, G. Zollner, and E. D. Rowton. 2011. Imidacloprid as a potential agent for the systemic control of sand flies. Journal of Vector Ecology 36: S148-S156.
Tsurim, I., Wasserberg, G. Ben Natan, and Z. Abramsky. 2020a. Systemic Control of Cutaneous Leishmaniasis Sand-Fly Vectors: Fipronil-Treated Rodent Bait Is Effective in Reducing Phlebotomus papatasi (Diptera: Psychodidae) Female Emergence Rate From Rodent Burrows. J Med Entomol.
Tsurim, I., Wasserberg, G. Ben Natan, and Z. Abramsky. 2020b. The Potential of Systemic Control of Sand Flies Using Fipronil in the Novel Leishmania major (Kinetoplastida: Trypanosomatidae) Reservoirs Meriones tristrami (Rodentia: Muridea) and Meriones crassus (Rodentia: Muridea). J Med Entomol.
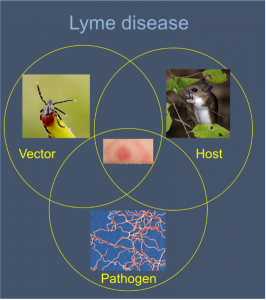
With an estimated rate of 300,000 cases per year, Lyme Borreliosis (LB) (also known as Lyme Disease [LD]) has become the primary vector-borne disease in the United and the fifth Nationally Notifiable disease. Lyme disease cases are concentrated in the Northeast and upper Midwest. However, during the last decade a clear southwesterly expansion of LB and its blacklegged tick vector (Ixodes scapularis) has been documented along Virginia’s eastern Appalachian foothills with current epidemic wave-front hovering just north of the North Carolina – Virginia border. Sporadic human cases have been described in north-western NC counties (Wake, Guilford, Haywood, Wilkes, Alleghany, and Buncombe). However, no information is available regarding the distribution and the abundance of the tick vector and rodent hosts and infection rates among them in these areas. During the last four years, we were funded by the North Carolina DHHS in order to conduct an active surveillance of the distribution of tick vector and the infection rates within their population. Using flagging, tick collection from hunted deer, and collection of ticks from small rodents, we detected a major cluster of tick and infection foci in the north western parts of NC and further work is being done in order to characterize the tick’s population structure, tick’s seasonal phenology and infection dynamics, and rodent host community structure and infection patterns.
For organisms lacking parental care and where larval dispersal is limited, oviposition site selection decisions are critical fitness-enhancing choices. The oviposition behavior of such insect vectors is a critical driver of their distribution and abundance. Therefore, improved understanding of this behavior is essential for our ability to control them. This is particularly important for the control of container breeding mosquitoes to which traditional broadcast spraying is typically not effective. It is, therefore, important to understand the response of gravid container-breeding mosquitoes to competitors, predators, and food resource level.
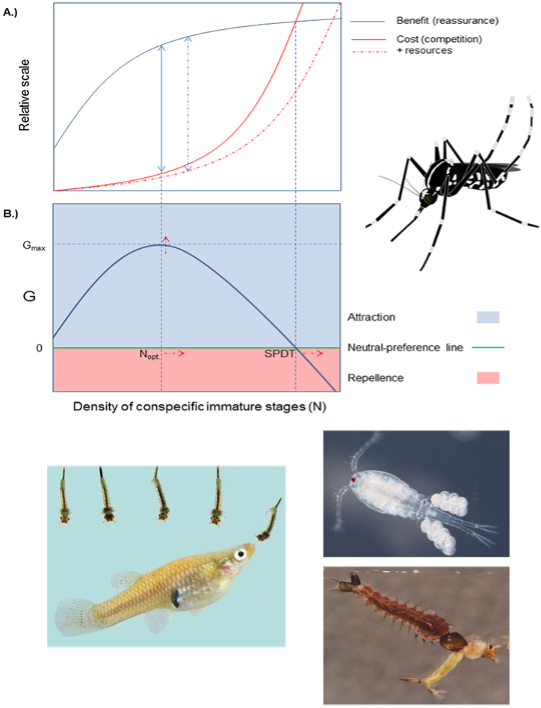
1. Hump-Shaped Density-Dependent Regulation of Mosquito Oviposition Site-Selection by Conspecific Immature Stages
The presence of conspecific immature stages in a potential oviposition site could, on the one hand, indicate the suitability of that site but on the other hand could indicate the potential for intraspecific competition. In this paper, we present a graphic model suggesting that the trade-off between these two opposing forces could result in a hump-shaped density-dependent relationship between oviposition rate and conspecific immature stage density (hereafter, the “Hump-shaped regulation model”) with positive effects of aggregation prevailing at low densities and negative effect of intraspecific competition prevailing at higher densities. We field-tested the predictions of this model at both the egg- and the larval levels with Aedes albopictus and evaluated if and how these relationships are affected by resource enrichment.
2. Oviposition site selection in Aedes albopictus (Diptera: Culicidae): effects of predation risk and food levels.
In this study, we evaluated the effect of food level on the oviposition behavior of Aedes albopictus in the presence or the absence of a nonlethal predator (caged dragonfly nymph). We also attempted to quantify the perceived cost of predation to ovipositioning mosquitoes. Mosquitoes were presented with oviposition cups containing four levels of larval food (fermented leaf infusion) with or without a caged libellulid nymph. By titrating larval food, we estimated the amount of food needed to attract the female mosquito to oviposit in the riskier habitat. As expected, oviposition rate increased with food level and decreased in the presence of a predator. However, the effect of food level did not differ between predator treatments. By calculating the difference in the amount of food for points of equal oviposition rate in the predator-present and predator-absent regression lines, we estimated the cost of predation risk to be 1950 colony-forming units per milliliter. In additional studies we showed that Gambusia fish has a significant deterrent effect on egg deposition but copepods and Toxorhynchites rutilus (predatory mosquito larvae) had no effect.
Wasserberg, G., N. Bailes, C. Davis, and K. Yeoman. 2014. Hump-Shaped Density-Dependent Regulation of Mosquito Oviposition Site-Selection by Conspecific Immature Stages: Theory, Field Test with Aedes albopictus, and a Meta-Analysis. PLos ONE 9
Wasserberg, G., L. White, A. Bullard, J. King, and R. Maxwell. 2013. Oviposition Site Selection in Aedes albopictus (Diptera: Culicidae): Are the Effects of Predation Risk and Food Level Independent? Journal of Medical Entomology 50: 1159-1164.


