Phlebotomine sand flies (Diptera: Psychodidae) transmit protozoan parasites (Leishmania spp.), as well as bacteria (Bartonella bacilliformis) and Phleboviruses. Most significant are the human leishmaniases. With no vaccine to protect against the etiologic agent, restriction of exposure to sand fly bites is the most effective preventive measure. Transmission prevention mainly involves personal protection (e.g., repellents, insecticide-treated clothing or bed-nets) and residual spraying with insecticides. However, the efficacy of the latter is decreasing due to the evolution of insecticide resistance. Furthermore, residual insecticide sprays also affect a wide range of non-target arthropods. Hence, a more focused, targeted, and efficient control method is urgently needed. With the rise of insect vector resistance to chemical pesticides and their effect on non-target species, there is an urgent need for the development of environment-friendly biologically based and targeted vector-control approaches. Utilizing a biological component of the vector’s biology to work against itself is considered as a hallmark of an efficacious approach because it is usually species-specific and less susceptible for the development of resistance. In my research, I target potential weak links that could potentially break the transmission chain of the Leishmania pathogens.
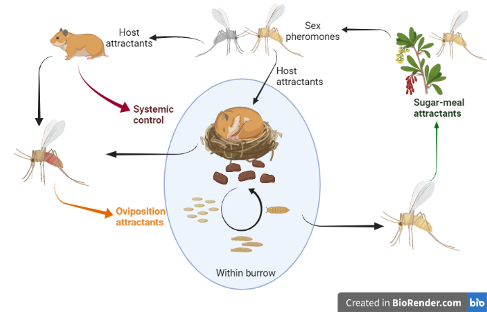
Life cycle of a sand fly species that is dependent on its blood-meal host for providing it with a breeding site and a shelter (termed – ‘tightly-coupled vector-host system’). Attractant-based intervention could target crucial aspects of its life cycle including sugar-meal attractants, sex pheromones, host cues, and oviposition attractants. My research (in this context) focuses, mainly, on oviposition attractants but also sugar-meal attractants. Feed-through systemic control uses the host to deliver the insecticide to the insect-host point of contact. This is also an important avenue my lab explores.

 An alternative approach to the delivery of the insecticide to the vector is to bring the vector to the insecticide using attractants. This attract-and-kill approach is commonly used to control disease vectors using volatile compounds such as sex pheromones, host odors, sugar meal sources, and bacterial mediated oviposition site attractants. In the context of controlling and surveilling disease vectors, oviposition-site attractants are expected to be particularly effective because they lure older females that have blood-fed at least once and are thus more likely to be infected with pathogens. Therefore, by targeting gravid females, control efforts can simultaneously reduce pathogen transmission and control population growth. Furthermore, since the response to oviposition cues is often species- or genus-specific, the impact on non-target species is expected to be minimal. For the same reason, the attractive lure can be integrated with a trap (e.g., CDC traps) for effective vector surveillance, providing a more precise estimate of the entomological risk of certain insect-borne pathogens in a given area. in addition, identifying oviposition stimulants can be important by facilitating sand fly mass rearing and transmission studies.
An alternative approach to the delivery of the insecticide to the vector is to bring the vector to the insecticide using attractants. This attract-and-kill approach is commonly used to control disease vectors using volatile compounds such as sex pheromones, host odors, sugar meal sources, and bacterial mediated oviposition site attractants. In the context of controlling and surveilling disease vectors, oviposition-site attractants are expected to be particularly effective because they lure older females that have blood-fed at least once and are thus more likely to be infected with pathogens. Therefore, by targeting gravid females, control efforts can simultaneously reduce pathogen transmission and control population growth. Furthermore, since the response to oviposition cues is often species- or genus-specific, the impact on non-target species is expected to be minimal. For the same reason, the attractive lure can be integrated with a trap (e.g., CDC traps) for effective vector surveillance, providing a more precise estimate of the entomological risk of certain insect-borne pathogens in a given area. in addition, identifying oviposition stimulants can be important by facilitating sand fly mass rearing and transmission studies.
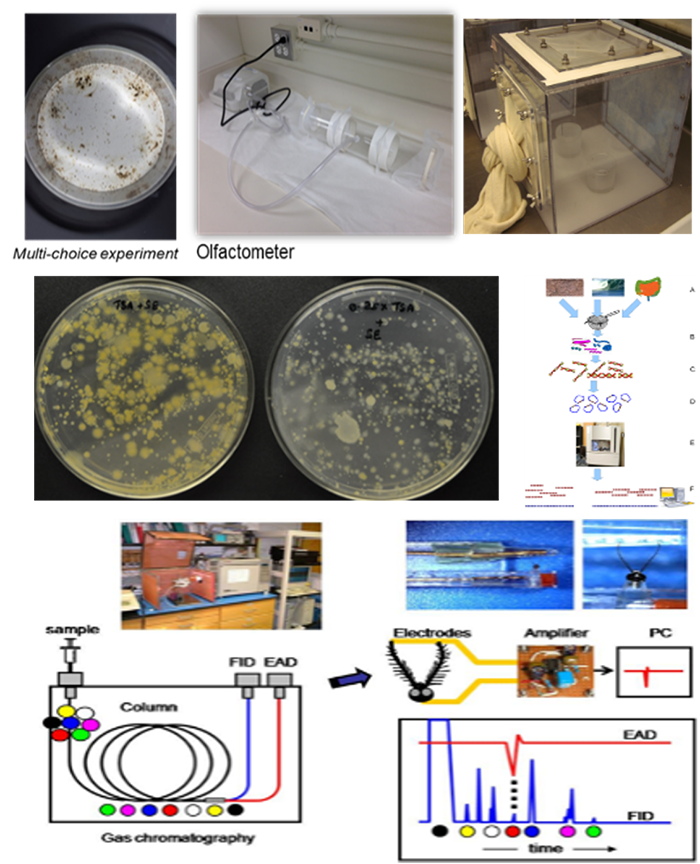
Unlike most biting Diptera, sand flies have a fully terrestrial life cycle. Eggs are typically laid in soil rich in organic material on which the coprophagic larvae feed and develop through four instars before pupation and adult emergence. During the past five years of an NIH-R01 funded, using an interdisciplinary project combining behavioral, electrophysiological, and microbiological studies (See, figure on the right), we made substantial contributions in advancing our understanding of sand fly oviposition biology. Among others, we optimized screening protocols of oviposition attractants and stimulants and characterized oviposition periodicity. Based on these bioassays, we identified a subset of highly attractive bacterial isolates, identified a range of highly attractive and oviposition stimulating compounds from bacterial and conspecific sources, characterized visual oviposition cues, described spatial skip oviposition of individual flies, identified egg hatching cues, and quantified the cost of autogenous oviposition.
A 3-chamber in-line olfactometer designed for use with sand flies was used as a high-throughput method to screen honeys for attractiveness to Phlebotomus papatasi (four geographic isolates), P. duboscqi (two geographic isolates), and Lutzomyia longipalpis maintained in colonies at the Walter Reed Army Institute of Research. A diversity of unifloral honey odors were evaluated as a proxy for the natural floral odors that sand flies may use in orientation to floral sugar sources in the field. In the 3-chamber in-line olfactometer, the choice modules come directly off both sides of the release area instead of angling away as in the Y-tube olfactometer. Of the 25 honeys tested, five had a significant attraction for one or more of the sand fly isolates tested. This olfactometer and high-throughput method has utility for evaluating a diversity of natural materials with unknown complex odor blends that can then be down-selected for further evaluation in wind tunnels and/or field scenarios.
 The strong dependency of some vectors on their host as a source of habitat can be viewed as a weak link in pathogen’s transmission cycles using the vertebrate host as a ‘Trojan horse’ to deliver insecticides directly to the vector-host point of contact (hereafter ‘feed-through systemic control’). This could, simultaneously, affect the survival of blood-feeding females and coprophagic larvae. Sand-flies, vectors of leishmaniasis worldwide, are often dependent on their bloodmeal host as a source of habitat and may therefore be good candidates for systemic control. In a preliminary collaborative study, we showed that Imidacloprid treated food pellets are effective in reducing larval survival that fed on treated rodent’s feces and on female feeding on treated blood. In a collaborative study with Dr. Ido Tsurin from the Ben-Gurion University, Israel, we deployed fipronil-treated baits in Meriones crassus burrows and demonstrated 86% reduction in the abundance of female sand-flies that exit burrows of Fipronil-treated jirds. We then, tested this approach at a larger town-level scale in the Negev desert of Israel, and demonstrated that this intervention significantly reduced L. major transmission rate.
The strong dependency of some vectors on their host as a source of habitat can be viewed as a weak link in pathogen’s transmission cycles using the vertebrate host as a ‘Trojan horse’ to deliver insecticides directly to the vector-host point of contact (hereafter ‘feed-through systemic control’). This could, simultaneously, affect the survival of blood-feeding females and coprophagic larvae. Sand-flies, vectors of leishmaniasis worldwide, are often dependent on their bloodmeal host as a source of habitat and may therefore be good candidates for systemic control. In a preliminary collaborative study, we showed that Imidacloprid treated food pellets are effective in reducing larval survival that fed on treated rodent’s feces and on female feeding on treated blood. In a collaborative study with Dr. Ido Tsurin from the Ben-Gurion University, Israel, we deployed fipronil-treated baits in Meriones crassus burrows and demonstrated 86% reduction in the abundance of female sand-flies that exit burrows of Fipronil-treated jirds. We then, tested this approach at a larger town-level scale in the Negev desert of Israel, and demonstrated that this intervention significantly reduced L. major transmission rate.