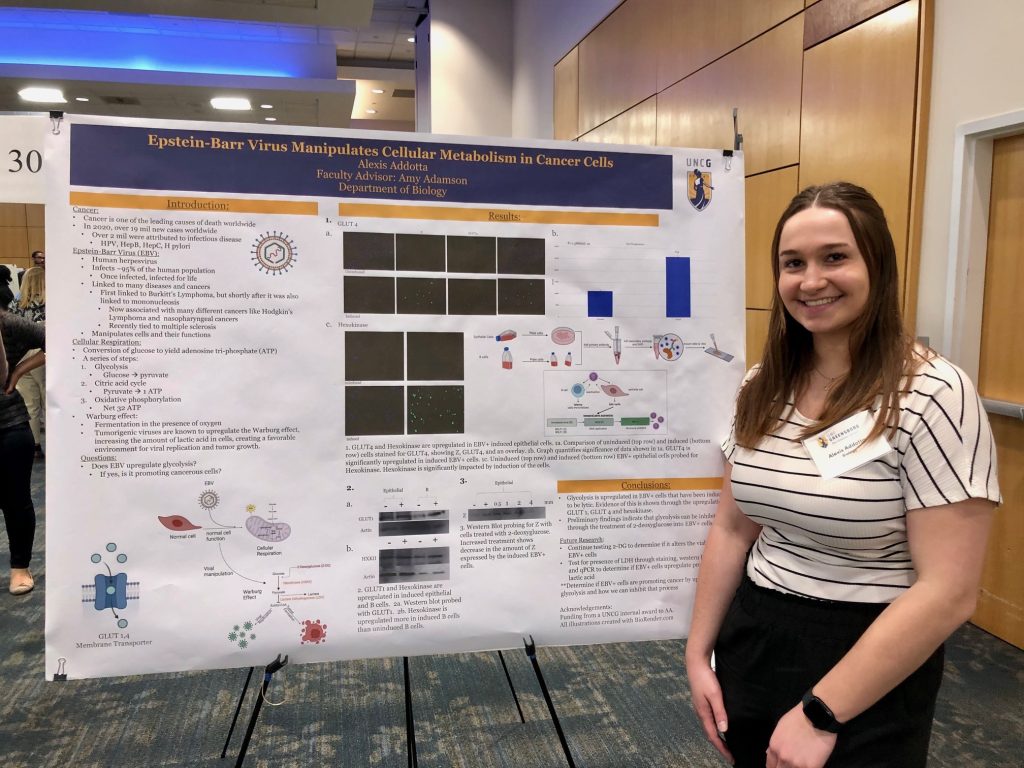
My research group is focused on intracellular virus-host interactions. We study how viruses affect cell biology at the molecular scale ~ most often viral protein-cell protein interactions and consequences. We are currently studying the ubiquitous Epstein-Barr and Influenza A viruses.
See the section below for our current lines of research.
Research

Epstein-Barr virus (EBV), an oncovirus, has been shown to promote cancer cell metastasis – including such features and cell motility, migration, and invasiveness. We have demonstrated that lytic (but not latent) EBV-infected epithelial cells will migrate into an open space. High levels of active cellular Mnk1 have also been correlated to increased metastasis. As we have already shown that EBV lytic replication activates high levels of Mnk1, we are now studying how EBV lytic replication and Mnk1 work together to promote the migration of EBV-positive cells. We demonstrated that inhibition of Mnk1 prevents migration of lytic cells, and are now dissecting out the mechanism by which EBV lytic proteins and Mnk1 cooperate to drive metastasis.

For efficient viral replication, numerous different families of viruses, including Herpesviruses, have evolved the ability to manipulate cellular metabolism. Several Herpesviruses are known to upregulate glucose uptake as well as the progression of glycolysis, a state known as aerobic glycolysis. This state is also a hallmark of many cancers (where it is also known as the Warburg effect), and such metabolic reprogramming may allow for increased cell growth, migration, invasion, and transformation of cancer cells. Aerobic glycolysis is typically accompanied by increased production of lactate, which can also dampen the cellular immune response against viral infection. Our lab is interested in how viruses manipulate glycolysis and the effects of such metabolic reprogramming.
We have new funding to support these studies:
“Epstein-Barr virus upregulates glycolysis to promote viral replication and persistence,” PI, UNCG Internal Funding Program. 1/1/2023 – 6/30/2024

Stemming from our mTOR work, we have identified Mnk1/2 (MAPK-interacting kinase 1/2) as an interesting cellular modulator of EBV lytic replication. This kinase, activated by the MAPKs ERK and p38, is best known for its promotion of cap-dependent translation through phosphorylation of the translation initiation factor eIF4E. We have found, that in addition to this function, Mnk1/2 participates in the regulation of EBV lytic replication, insomuch as Mnk1/2 becomes highly phosphorylated/activated during early lytic replication within EBV-infected cells, and that treatment with a Mnk inhibitor alters subsequent lytic replication – in a cell-type dependent manner. Mnk1/2, a known mediator of metastasis of cancer cells, also plays a role in the migration of lytic EBV-infected epithelial cells. All in all, this makes Mnk1/2 an interesting cellular protein to focus on in terms of suppressing EBV lytic replication and metastatic potential.

Epstein-Barr virus (EBV), a member of the Herpesviridae family, is a widespread pathogen that is associated with a variety of diseases, including infectious mononucleosis, several types of cancers, and multiple sclerosis. Due to its potential health-related effects, it is important to study this virus at the molecular level, including how EBV viral proteins interact with, both directly and indirectly, cellular proteins, especially cellular proteins whose functions are known to be associated with diseases such as cancer. Our long term goal for this line of research is to discover and study important viral/cellular protein interactions, with the hope to manipulate these interactions to prevent viral replication.
With this research we aim to investigate the interaction of mTOR pathway-related proteins with the EBV lytic cycle. mTOR (mechanistic target of rapamycin) is a kinase at the heart of a major signaling pathway. Extracellular signals such as various nutrient levels and growth factors impinge on the mTOR pathway to control a variety of processes including protein translation, autophagy, cell growth, and mitochondrial metabolism. We previously found that the inhibition of mTOR was sufficient to reduce EBV lytic replication in B cells, while having the opposite effect in epithelial cells. (1) By determining how the mTOR pathway and EBV intersect, we will gain insight into the consequences of giving mTOR inhibitors to patients for immunosuppressive or anti-cancer applications, in relation to EBV infection status.
Adamson, A., Jeffus, D., Davis, A., and Greengrove, E. 2022. Epstein-Barr virus lytic replication activates and is dependent upon MAPK-interacting kinase 1/2 in a cell-type dependent manner. Virology 572: 72222-85.
Adamson, A., B. Le, and B. Siedenburg. 2014. Inhibition of mTOR inhibits lytic replication of Epstein-Barr virus in a cell-type specific manner. Virology Journal 11:110.
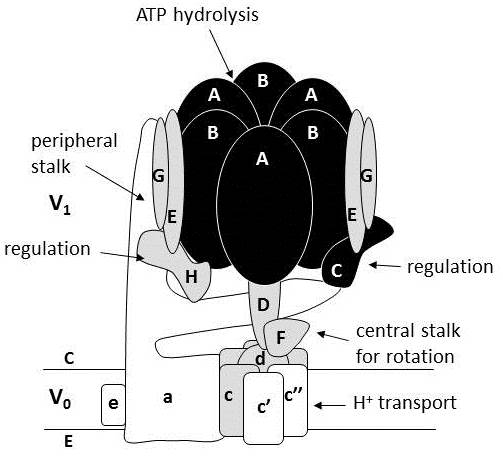
As new influenza virus strains emerge, finding new mechanisms to control viral infection is imperative. We have found that we can control influenza infection of mammalian cells by altering the level of glucose given to cells. Higher glucose concentrations induce a dose-specific increase in influenza infection. Linking influenza virus infection with glycolysis (the first stage of glucose metabolism), we found that viral replication was significantly reduced after cells were treated with chemical inhibitors of glycolysis. The addition of extracellular ATP after glycolytic inhibition restores influenza infection. We have also determined that higher levels of glucose promoted the assembly of the vacuolar-type ATPase (a proton pump), and increased vacuolar-type ATPase proton-transport activity. We found that the increase of viral infection via high glucose levels could also be reversed by inhibition of this proton pump, linking glucose metabolism, vacuolar-type ATPase activity, and influenza viral infection. Taken together, we propose that altering glucose metabolism may be a potential new approach to inhibit influenza viral infection. (1)
In addition, we are targeting specific subunits of the proton pump, to fine tune-inhibition of Influenza virus infection. We find that the most useful approach to limiting the proton pump’s activity, while maintaining cellular functioning, is to knockdown levels of the subunit V1A. (2)
Adamson, A. 2020. Knockdown of the V1A subunit of the vacuolar V1V0-ATPase proton pump reduces infection by Influenza A virus in a glucose dependent manner. Under review.
Kohio, H. and A. Adamson. 2013. Glycolytic Control of Vacuolar-Type ATPase Activity: A Mechanism to Regulate Influenza Viral Infection. Virology 444: 301-309.
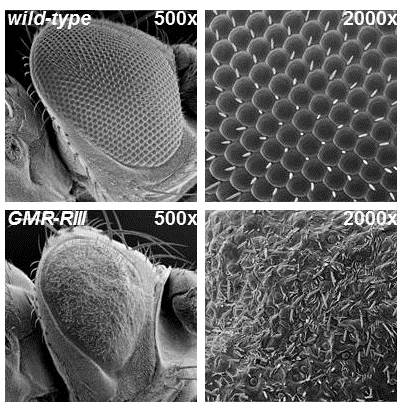
We have successfully utilized Drosophila melanogaster as a model system to identify and study viral protein- host protein interactions. We have studied Epstein-Barr virus and Influenza A virus proteins with this system, and identified important cellular mediators (and potential drug targets) of viral infection.
Drosophila model publications:
- Adamson, A., N. Wright, and D. LaJeunesse. 2005. Modeling Early Epstein-Barr Viral Infection in Drosophila melanogaster: The BZLF1 Protein. Genetics 171:1125-1135.
- Adamson, A., Chohan, K., Kincaid, J., and LaJeunesse D. 2011. A Drosophila Model for Genetic Analysis of Influenza Viral/Host Interactions. Genetics 189: 495-506.
- Adamson, A., and LaJeunesse D. 2012. A Study of Epstein-Barr Virus BRLF1 Activity in a Drosophila Model System. The Scientific World Journal – Cell Biology Domain 2012:1-9.
Publications stemming from Drosophila work:
- Adamson, A. 2005. Epstein-Barr virus BZLF1 protein binds to mitotic chromosomes. Journal of Virology 79:7899-7904.
- Adamson, A. 2013. Identification of an N-acetylglucosaminyltranferase-IV as a modifier of Epstein-Barr virus BZLF1 activity. Open Journal of Genetics 3(1) 1-5.
- Kohio, H. and A. Adamson. 2013. Glycolytic Control of Vacuolar-Type ATPase Activity: A Mechanism to Regulate Influenza Viral Infection. Virology 444: 301-309.
- Adamson, A., B. Le, and B. Siedenburg. 2014. Inhibition of mTOR inhibits lytic replication of Epstein-Barr virus in a cell-type specific manner. Virology Journal 11:110.
- Adamson, A. 2020. Knockdown of the V1A subunit of the vacuolar V1V0-ATPase protein pump reduces infection by Influenza A virus in a glucose dependent manner. Under review.
- Adamson, A., Jeffus, D., Davis, A., and Greengrove, E. 2021. Epstein-Barr virus lytic replication activates and is dependent upon MAPK-interacting kinase 1/2 in a cell-type dependent manner. Under review.
More ongoing!