Humanity is facing an epidemiological transition, characterized by the resurgence and emergence of old and novel infectious diseases the majority of which are associated with a wildlife source (zoonoses) and/or arthropod vectors. Human-induced environmental changes such as encroachment into pristine environments, land-use change, and climate change has been implicated as key drivers of this phenomenon. For a transmission hot spot to become established, the ecological niches of the pathogen, host, and (for vector borne pathogens) the vector must overlap within a permissive environment. The specific subset of environmental conditions that enable the persistence of the transmission cycle is often referred to as the “disease niche” (figure below). Human encroachment into pristine environments may exposes humans to novel pathogens but it may also enhance transmission by improving the ecological conditions for some (or all) the ecologic components of the enzootic system. We showed that such small-scale anthropogenic effects have the potential to enhance transmission dynamics for both Old-World Cutaneous Leishmaniasis in Israel and for La Crosse Encephalitis in western North Carolina. However, large scale anthropogenic landscape modification may also have substantial effects on the structure and function of these systems. However, these are currently poorly understood.
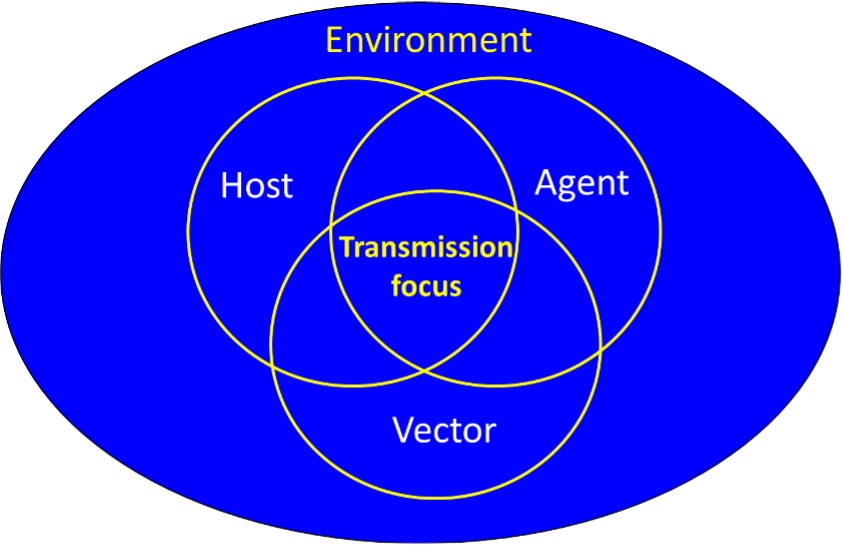
The disease niche conceptual model
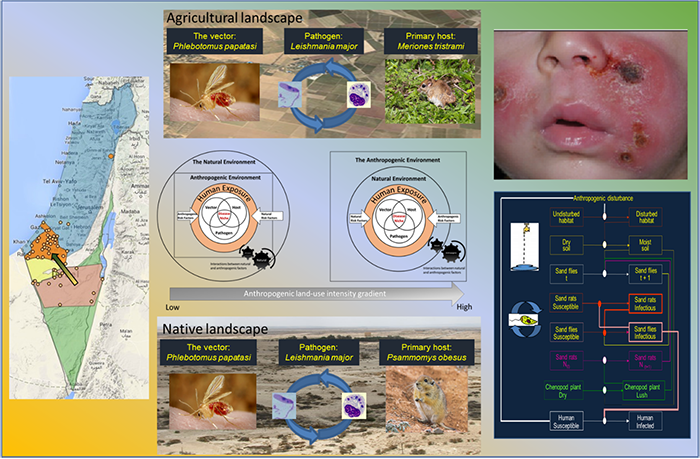
Leishmaniases are the third most pervasive insect-bone diseases (following Malaria and Dengue), affecting tropical as well as arid regions in under-developed countries. However, CL is a neglected disease and developing a more thorough understanding of its ecology is vital. We are using Old World Zoonotic Cutaneous Leishmaniasis (ZCL) as a model system to study this question. Leishmania major, the protozoan parasite that causes Old World ZCL, is transmitted by the sand fly Phlebotomus papatasi and maintained by Psammomys obesus (the fat sand rat) in native arid landscapes but recently was described to also be maintained by Meriones tristrami in agriculturally modified regions. In native arid regions, we have shown that anthropogenic disturbances, of various types, share the common effect of elevating soil moisture, which enhances sand fly and sand rat abundance and thereby amplifying pathogen transmission and spill-over rates of the infection into adjacent human populations (see, flow chart model below) (Wasserberg et al. 2002, 2003ab, Berger et al. 2014). However, a recent outbreak of Old World ZCL in a northern, mostly agricultural semi-arid region, is yet poorly understood. We are currently studying this system in order to better understand the ecological and, possibly, evolutionary processes underlying this emergence.
 La Crosse encephalitis (LACE) is a neurodegenerative viral pediatric disease transmitted by Aedes mosquitoes in the Appalachian mountain range. It is characterized by a highly focal distribution with vector population being highly fragmented given the topographic nature of the area. The incidence of LACE has increased in the greater Appalachian region G. Wasserberg in Collaboration with B. Byrd from Western Carolina University are researching the putative causal association between anthropogenic landscape change and LACE emergence. In a previous study, this work showed that poor peridomestic conditions enhance LACE risk by modifying the habitat use patterns of its mosquito vectors (one domestic: Aedes triseriatus and two invasives: A. japonicus and A. albopictus; Tamini et al. 2021). Current studies are evaluating the mosquito and virus distribution along forest-to-field ecotones (the predominant landscape feature in this region) and tested experimentally the effect of tire introduction on the distribution and population dynamics of the disease vectors at the horizontal and vertical dimensions (Schwarz 2021). Importantly, work has shown that vertical habitat features (i.e., tree canopy height) are important in this vector system (Schwarz et al. 2020).
La Crosse encephalitis (LACE) is a neurodegenerative viral pediatric disease transmitted by Aedes mosquitoes in the Appalachian mountain range. It is characterized by a highly focal distribution with vector population being highly fragmented given the topographic nature of the area. The incidence of LACE has increased in the greater Appalachian region G. Wasserberg in Collaboration with B. Byrd from Western Carolina University are researching the putative causal association between anthropogenic landscape change and LACE emergence. In a previous study, this work showed that poor peridomestic conditions enhance LACE risk by modifying the habitat use patterns of its mosquito vectors (one domestic: Aedes triseriatus and two invasives: A. japonicus and A. albopictus; Tamini et al. 2021). Current studies are evaluating the mosquito and virus distribution along forest-to-field ecotones (the predominant landscape feature in this region) and tested experimentally the effect of tire introduction on the distribution and population dynamics of the disease vectors at the horizontal and vertical dimensions (Schwarz 2021). Importantly, work has shown that vertical habitat features (i.e., tree canopy height) are important in this vector system (Schwarz et al. 2020).