
Esther Leise's Web Establishment
 |
Esther Leise's Web Establishment
|
 |
Home |
Research Projects |
Publications |
Courses |
UNCG Home Page |
Biology Home Page |
Other Links |
CURRENT RESEARCH PROJECTS AND POTENTIAL FOR STUDENT INVOLVEMENT:
We also plan to demonstrate the presence of GABAergic neurons in the CNS of larval Ilyanassa with immunocytochemistry. This future project is suitable for both graduate and undergraduate collaboration. If you are interested in working in my laboratory, contact me by email, or come to my office in 427 Eberhart Bldg.
|
|
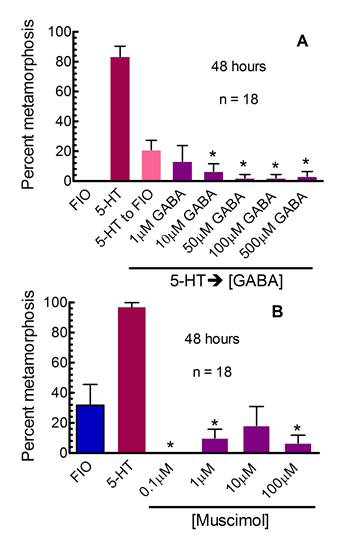
CURRENT STUDENT PROJECT: Dhani Biscocho – degree expected 2012. Dhani is currently conducting bath application and injection experiments whose results should support our hypothesis that GABA acts as an inhibitory neurotransmitter in the circuit that controls metamorphosis in larval Ilyanassa. To the right are examples of data we have collected that demonstrate GABAergic inhibition of metamorphosis. In (A), larvae were exposed to 100µM serotonin (5-hydroxytryptamine or 5-HT) for 5 hours, then incubated in GABA at the indicated concentrations (5-HTàGABA). Multiple concentrations of GABA significantly reduced levels of metamorphosis (*) compared to the 5-HT to FIO control. In (B) the GABAA agonist muscimol inhibited metamorphosis after larvae were incubated in it for 48 hours. As larvae age in culture, they begin to metamorphose spontaneously, in the absence of any known inducer. They displayed such behavior in this experiment. Muscimol significantly inhibited (*) high levels of spontaneous metamorphosis (compare FIO in A and B) seen in this experiment. (Χ2= 6.635, α=0.01, corrected for multiple comparisons, n is # of replicates, with 5 animals per replicate, from Leise et al., 2009, Soc. Neurosci. Abstr. Viewer & Itinerary Planner. Program 889.2).
|
 |
PREVIOUS STUDENT PROJECTS: In addition to the graduate students mentioned below, numerous (~36 to date) undergraduates have collaborated with us on various aspects of these projects. |
|
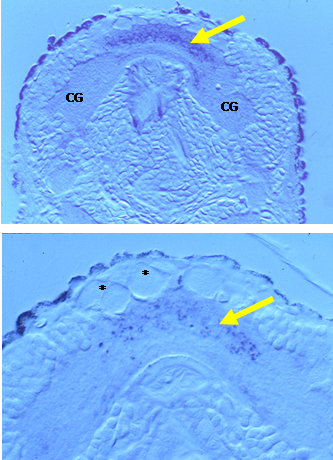
Miao-Fang Lin Althshuller - MS degree 1995: Nitric oxide synthase (NOS) is the enzyme that produces the gaseous neurotransmitter nitric oxide (NO). Neuronal (n)NOS is a form of NADPH diaphorase (NADPHd) and thus histochemical detection can be used to localize nNOS. Miao-Fang conducted NADPH diaphorase histochemistry on larval, juvenile and metamorphosing Ilyanassa and discovered NADPH diaphorase activity in all ganglia of the larval nervous system. The ganglion with the highest activity was the apical ganglion (AG), which is lost at metamorphosis. To the right are two micrographs of sections through a competent (top) and a metamorphosing (bottom) larva. Both transverse sections pass through the AG. At the top, extensive staining is indicative of NADPH diaphorase (yellow arrow) and thus NOS activity in the neuropil of this ganglion. In metamorphosing larvae, NADPHd activity has decreased, as evidenced by the less intense punctate blue staining in a similar region (yellow arrow), below the cellular remnants of the apical ganglion (asterisks, modified from Lin and Leise, 1996, J. comp Neurol. 374:180-193 and 194-203). The AG is enlarged in this image, compared to the one above it. CG marks the neuropil of a cerebral ganglion. The dramatic change in the activity of NOS, as indicated by this histochemical method, led us to speculate that NO might have some important role in the control of metamorphosis in this animal. This work led directly to pharmacological experiments conducted by Stephan Froggett. |
 |
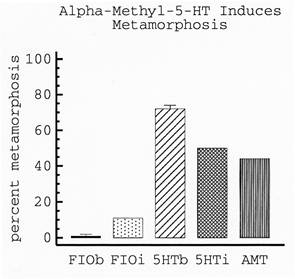
| Julia Couper Leo - MS degree 1995: The neurotransmitter serotonin (5-HT) was reported to induce metamorphosis in larval Ilyanassa (Levantine and Bonar, 1986, American Zoologist 26: 14A). However, the mode of action of 5-HT was unclear. In other words, was 5-HT inducing metamorphosis by binding to external chemosensory receptors or by being take up by larvae to act internally as a neurotransmitter? To begin to distinguish between these 2 possibilities, Julia conducted a series of pharmacological studies including both bath application and injection experiments. Her results with various serotonergic reagents provided evidence supporting the idea that 5-HT acts as a neurotransmitter in the circuit that promotes metamorphosis (from Couper and Leise, 1996, Biological Bulletin 191:178-186). The graph to the right demonstrates that injection of serotonin (5HTi) induces far more larvae to metamorphose than does injection of artificial seawater (FIOi). Injections of the 5-HT agonist, α-methyl 5-HT (AMT), also induce larval metamorphosis. Results illustrated in this graph are 48 hours after injection. |
 |
| Stephan Froggett - MS degree 1997: To determine whether nitric oxide (NO) might suppress or promote metamorphosis, Steve conducted a series of pharmacological investigations using nitrergic reagents. Bath application of NO-donors to competent larvae elicited no responses, as did most NOS inhibitors. However, when injected into larvae, several NOS inhibitors induced metamorphosis, suggesting that NO functions to suppress metamorphosis during the larval stage (from Froggett and Leise, 1999, Biological Bulletin 196:57-62). An example of his data are to the right. In this experiment, N-methyl-L-arginine acetate (L-NMMA), a potent NOS inhibitor, induced significantly more larvae to metamorphose, within 24 hours of injection, than did FIOi. 5-HT and injected 5-HT (i5-HT) were additional controls in this experiment. Steve’s experiments demonstrated a physiological role for NO in molluscan larvae, but didn’t tell us where the nitrergic neurons might be located. Supported in part by NSF grant IBN-9604516. |
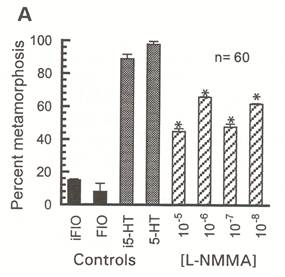 |
| Keow Thavaradhara Essig - MS degree 2000: To determine where NO-producing neurons in larval Ilyanassa might be located, Keow conducted immunocytochemistry on competent and pre-competent larvae. Her results indicated that most neurons of the apical ganglion display NOS-like immunoreactivity (NOS-IR), meaning that they most likely contain nNOS and thus produce NO. During larval development, the number of NOS-IR neurons increases. The micrograph to the right shows a head of a competent larva with NOS-immunoreactive neurons. (modified from Thavaradhara and Leise, 2001, Journal of Neurocytology 30(6):449-456). Supported in part by NSF grant IBN-9604516. |
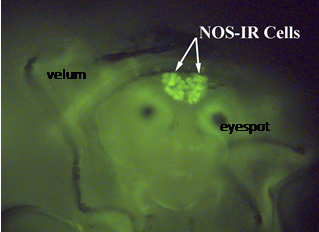 |
| Nathaniel Durham - MS degree 2002: The classical target for NO is the intracellular second messenger cGMP. To determine if NO in larval Ilyanassa act in a cGMP-dependent fashion, Nathan conducted several different types of investigations, including pharmacological experiments, radioimmunoassays, enzyme-linked immunoassays and immunocytochemistry (Leise et al., 2004, American Zoologist 41:258-267). A number of Nathan’s experiments were successful and demonstrated that by modulating larval levels of cGMP, one could affect levels of metamorphosis. One such example is depicted in the graph to the right. Here, NS-2028, an inhibitor of soluble guanylyl cyclase activity, promoted serotonergically-induced metamorphosis after 24 hours of incubation (from Durham, 2002, M.S. Thesis, UNCG). Supported by NSF grant IBN-9604516. |
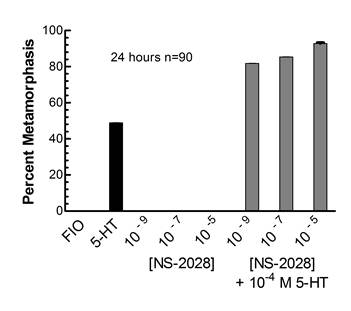 |
| David Gifondorwa - MS degree 2002: David determined that loss of the apical ganglion in larval Ilyanassa obsoleta occurs through a form of programmed cell death (PCD) that could be triggered by decreased production of nitric oxide. He used several anatomical techniques in his investigations, include Hoechst 33342 staining, the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay and confocal microscopy. The TUNEL assay identifies nuclei with fragmented DNA, a situation that occurs during PCD. C and D, to the right, are two adjacent sections from larvae with partial velar lobes, 24 hours after induction with 5-HT. Arrows indicate TUNEL-positive nuclei (in C) that also stain intensely with Richardson’s method (in D). Below these are sections from a larval with intact velar lobes, also at 24 hours after induction of metamorphosis, but this time with 7-Nitroindazole, a NOS inhibitor that works well in bath application. Even before loss of the velar lobes is visible, the apical ganglion has begun to distintegrate. (H) is a section stained by Richardson’s method which displays several large cellular remnants in the apical ganglion (asterisks). In some, the remains of a highly condensed nucleus is still detectable. An adjacent section (G) was stained with Hoechsts 33342 dye and viewed under the confocal microscope. This stain should show a distinct color shift in nuclei with fragmented DNA. No cells in the AG displayed this color shift, and because so few nuclei even stained with Hoechst’s dye, David concluded that by 24 hours, induced larvae were often well past the initial stages of programmed cell death (modified from Gifondorwa and Leise, 2006, Biological Bulletin 210(2):109-120). Supported by NSF grants IBN-0130677 and DBI-0319021 and NOAA Sea Grant mini-grant 1998-0617041. |
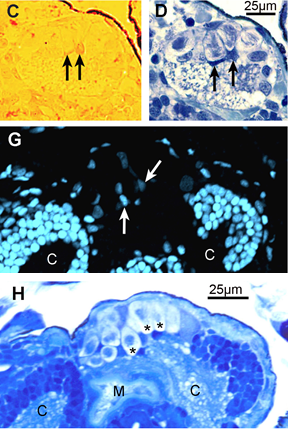 |
Brian Burrows - MS degree 2005: Work by both Miao-Fang Lin Altshuller and Keow Thavaradhara Essig had demonstrated that NO was present in larval brains during development. Because NO has been shown to control neurogenesis by suppressing DNA synthesis in other invertebrates, we hypothesized that NO might have this same role in larval Ilyanassa. Thus, Brian investigated this idea, that larval neurogenesis (production of new neurons) might be controlled by NO. To determine how rates of neurogenesis change during larval development, Brian incubated larvae at a number of ages in 5-bromo-2’-deoxyuridine (BrdU) for 3 hours, to label cells that were undergoing replication, and presumably, mitosis. He then localize BrdU labelling by immunocytochemistry. He stained all nuclei in these specimens with 4’,6-diamidino-2-phenylindole (DAPI) to facilitate counting all neuronal nuclei and compared that number to BrdU-labelled nuclei in 9 major ganglia. As indicated in the graph to the right, Brian determined that levels of neurogenesis drop as development progresses, which may be correlated with changes in NO levels (from Hens et al., 2005, Soc. Neurosci. Abstr. Viewer & Itinerary Planner CD-ROM. Program 30.11). Further experiments to directly link NO production to neurogenesis are in progress. Supported by NSF grants IBN-0130677 and DBI-0319021. |
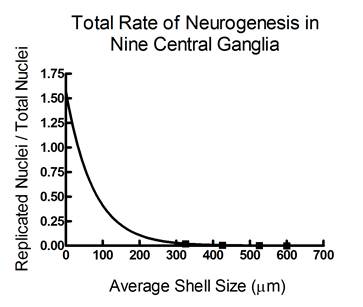 |
| Kenneth Fowler – MS degree 2005: Work by a number of previous graduates students, using a variety of methods, had generated much correlative data suggesting that NO levels should drop once metamorphosis was triggered. To gain direct molecular evidence in support of this idea, Ken cloned a portion of the larval neuronal NOS gene. Through reverse transcription-based polymerase chain reaction (RT-PCR) analysis, as shown to the right, Ken discovered that levels of NOS gene expression decrease during the 24 hours following metamorphic induction (modified from Hens et al., 2006, Biological Bulletin 211(3):208-211). Ken worked with both Dr. Mark Hens and Dr. Leise while conducting his M.S. thesis research. Supported by NSF grant IBN-0130677 |
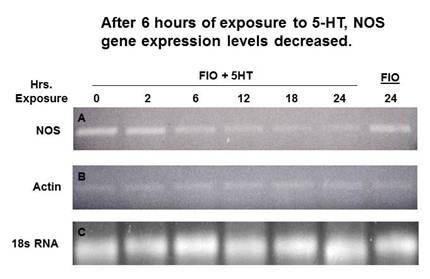 |
| Allison Weaver Deal – MS degree 2009: Allison developed an Ilyanassa-specific antibody to nitric oxide synthase (NOS), the enzyme that produces NO. As indicated in the graph to the right, her studies of the temporal expression of the NOS protein during metamorphosis confirmed Ken’s earlier RT-PCR results, showing that NOS protein levels decrease during metamorphosis. Her work suggested that NO production may be controlled by regulation of the quantity of NOS present as well as by the level of NOS enzyme activity. Allison also worked with both Dr. Mark Hens and Dr. Leise while conducting her M.S. thesis research. Supported in part by NSF grant IBN-0130677. |
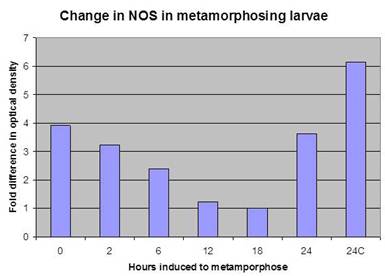 |