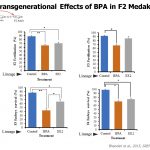
Research
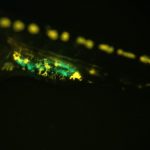
Sex differences in germ cells are believed to be epigenetically regulated, as distinct differences in DNA methylation and histone modifications have been found in germ cells during sex determination…
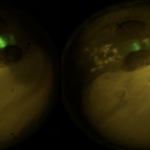
In model organisms, mate selection and mating behaviors have been found to be affected by developmental exposure to environmental estrogenic or anti-androgenic chemicals, suggesting that current…
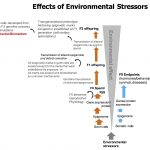
Gene-environment interactions can lead to emergence of phenotypes. Environmental stressors are able to induce epigenetic changes (chemical modifications on DNA structure) that are mitotically…
Growing number of studies reveal the fact that the aquatic environment is threatened by increasing rate of chemical contamination…